send link to app
A mobile application for Study participants. The app includes the following features:
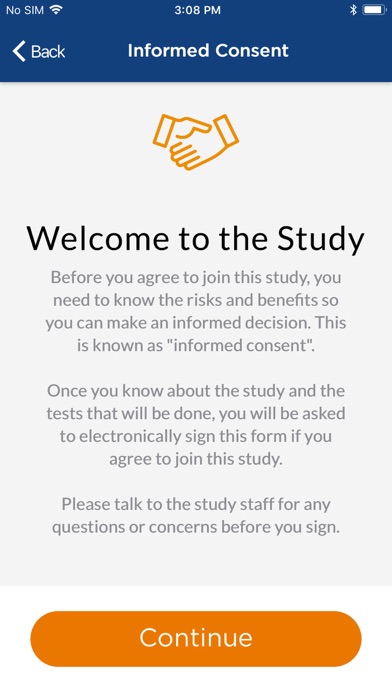
• Electronically sign informed consent form (ICF)
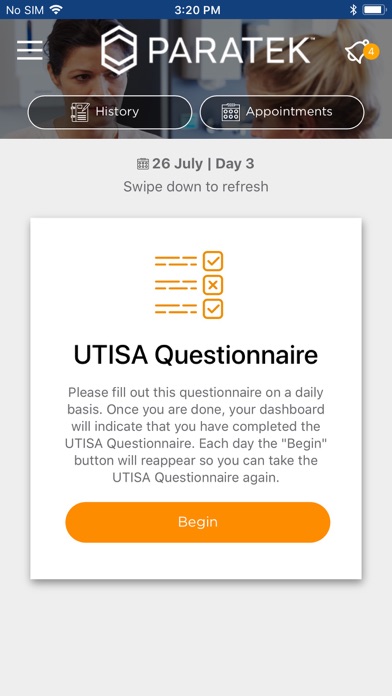
• Fill out a daily UTI Symptoms Assessment Questionnaire (UTISA Questionnaire)
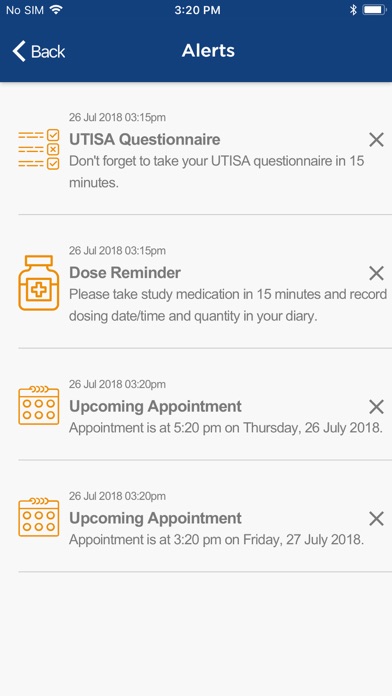
• Receive reminders for dosing, UTISA Questionnaire, and appointment
• View calendar with upcoming Study appointments
• Video tutorials and materials on how to use the app
• Study Site contact information
• Information about the Study